Our lead asset
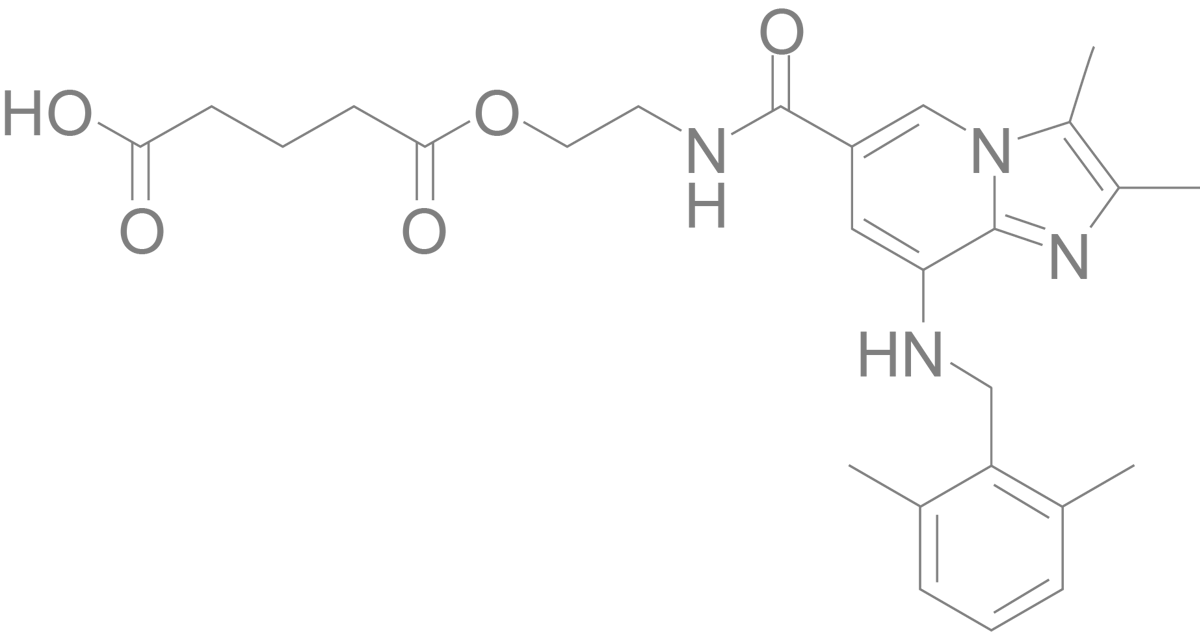
Linaprazan glurate
Our drug candidate represents a novel mode of action with an improved pharmacokinetic profile and the potential to have superior efficacy. Linaprazan glurate, a Potassium-Competitive Acid Blocker (P-CAB) is a prodrug of linaprazan, a substance initially developed and tested by AstraZeneca. The gastric acid control ability of linaprazan glurate has in studies shown to be superior to current medication.
Linaprazan glurate is a P-CAB and a prodrug of linaprazan, a substance initially developed and tested by AstraZeneca. Linaprazan glurate represents a new generation of acid blockers and therapy, building on world leading industrial tradition.
Linaprazan glurate is addressing a large unmet medical need, estimated to be over 20 million people in Europe and USA. The main target groups are patients with moderate to severe eGERD or Helicobacter pylori (H. pylori) infection, where available therapy is not effective enough. Linaprazan is the active metabolite in linaprazan glurate.
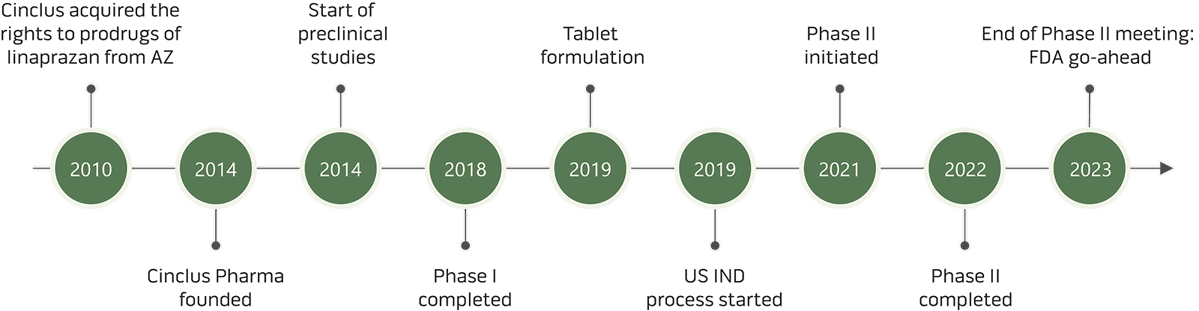
History and expected milestones
-
During 2001-2005 AstraZeneca performed Phase I and Phase II clinical studies on the product candidate linaprazan with the intention to develop the successor to Losec and Nexium. As not all patients receive sufficient symptom control and healing of erosions with existing medication, AstraZeneca identified an unmet medical need which led to the launch of a P-CAB development project.
-
Comprehensive data from 23 Phase I studies including more than 600 subjects exposed to linaprazan and two Phase II studies including approximately 2000 patients exposed to linaprazan shows that linaprazan was well tolerated, with a fast onset of action and full effect at first dose.
-
In 2005 AstraZeneca decided to close the linaprazan project. The rights of two molecules, so-called "prodrugs", of linaprazan were later acquired by two of the founders of Cinclus Pharma.
Read more about the company history
Linaprazan glurate shows promising results
The prodrug of linaprazan, linaprazan glurate has a longer plasma residence time in the body compared to linaprazan. It shows total control of gastric acid production and is tailored for patients with moderate to severe eGERD.
Clinical development in brief
The Phase I program is proceeding as planned. The Phase II study on eGERD was based on:
-
Precedent trials with >~2,600 subjects
-
Excellent Biomarkers for pH-level and clinical efficacy
-
A favorable low C-max predicts a strong safety profile
Read about the topline results
Read more about the clinical development of linaprazan glurate
Indication aspiration
The indication aspiration for linaprazan glurate is the treatment of erosive Gastroesophageal reflux disease (eGERD) in all grades A-D including healing, maintenance of healing, and relief of symptoms. The main target groups of linaprazan glurate are patients with moderate to severe eGERD (esophagitis or erosive GERD) and patients who do not get sufficient disease control with available therapy.
Patients:
-
with moderate to severe eGERD
-
with eGERD where PPIs are not sufficient in the need of improved symptom relief, healing erosions, and quality of life
-
with Helicobacter pylori (H. pylori)
Read more about the different stages of GERD
Linaprazan glurate is outperforming linaprazan
The prodrug linaprazan glurate has solved issues highlighted during the linaprazan project.
Issue 1 with linaprazan: Too short acid control
Linaprazan was quickly eliminated from the body and had too short duration of acid inhibition. To achieve long acid control linaprazan had to be overdosed. As a result, it was not possible to show superiority.
Solution:
This was accomplished with linaprazan glurate and shown in toxicological studies and in two Phase I studies
Issue 2 with linaprazan: Cmax levels too high
The resulting Cmax levels were on average 20 times higher than the level required for full inhibition of acid secretion, resulting in increases in liver transaminases in a few patients.
Solution:
Administration of linaprazan glurate results in approximately 75% lower C-max values compared to linaprazan, which minimizes the load on the liver.
A long duration effect is already accomplished with linaprazan glurate in toxicological studies and in two phase I studies. A solid oral formulation is in place and the next generation oral formulation is in development, expected to be finished by phase III.
Read more about the clinical development of linaprazan glurate
Mode of action P-CABs
Potassium-Competitive Acid Blocker (P-CAB)
P-CABs inhibit the proton pump but at a different site and through a different mechanism of action than proton pump inhibitors (PPIs). P-CABs block the potassium channel in the proton pump. They bind ionically, unlike PPIs, which bind covalently. This means that the binding action of P-CABs is reversible. These agents have a faster onset of action than PPIs, and they have a more flexible action in inhibiting acid secretion.
Source: https://www.gastroenterologyandhepatology.net
P-CABs like linaprazan glurate work differently from traditional PPI-based drugs like Losec and Nexium. Learn more about how they play a role in helping people to control acid secretion and manage GERD here.