
Clinical development
Follow the progress and further development of linaprazan glurate
Clinical development
The ongoing clinical development and testing of linaprazan glurate are showing promising results in acid control and in lowering the C-max value*, minimizing the load to the liver. Follow the progress and further development.
The Phase II study was initiated in Q3 2021 within a de-risked clinical development program based on results from Phase I studies and precedent trials on its active metabolite, linaprazan. Based on literature data and the results from the completed Phase I studies, excellent biomarkers lower the development risk moving forward. These biomarkers were applied in the design of the Phase II study, with the primary endpoint to optimize the precision of the dose selection for the Phase III studies. The LEED Phase II study was completed in Q4 2022, and "for patients with moderate to severe eGERD, LA grade C/D, the highest four-week healing rate in a linaprazan glurate dosing group was 89%, compared to 38% in the lansoprazole group." The first Phase III study, HEEALING 1 was initiated in September 2025.
Clinical trials on linaprazan glurate – Phase I to Phase III

Phase I
Phase I was initiated in February 2017 and recruitment in the "first-in-human study" was finalized in Q4 2017. Three Phase I clinical trials are completed. Additional Phase I studies are ongoing and planned. All studies so far showed that linaprazan glurate is safe and well tolerated.
Phase II
Phase II was initiated in H2 2021 within a de-risked clinical development program based on preceding trials with the active metabolite linaprazan and completed in Q4 2022 with successful results. The primary objective was to support the selection of the optimal dose for the Phase III studies based on dose-dependent healing rates of erosive esophagitis.
The program is based on:
-
Precedent trials that exposed ~2,600 subjects, to the active metabolite of linaprazan glurate
-
Excellent biomarker available
-
Strong correlation between plasma concentration and pH-control
Phase III
In August 2025 Cinclus Pharma received positive feedback from regulatory authorities regarding the first Phase III trial, HEEALING 1. The first patient in HEEALING 1 was screened in September 2025.
Studies performed on linaprazan (the main metabolite)
Comprehensive data from 23 Phase I studies that exposed more than 600 subjects and two Phase II studies that exposed approximately 2000 patients to the active metabolite show that linaprazan was well tolerated, with a fast onset of action and full effect at the first dose. Linaprazan is the active metabolite of linaprazan glurate.
Phase I – Three studies completed
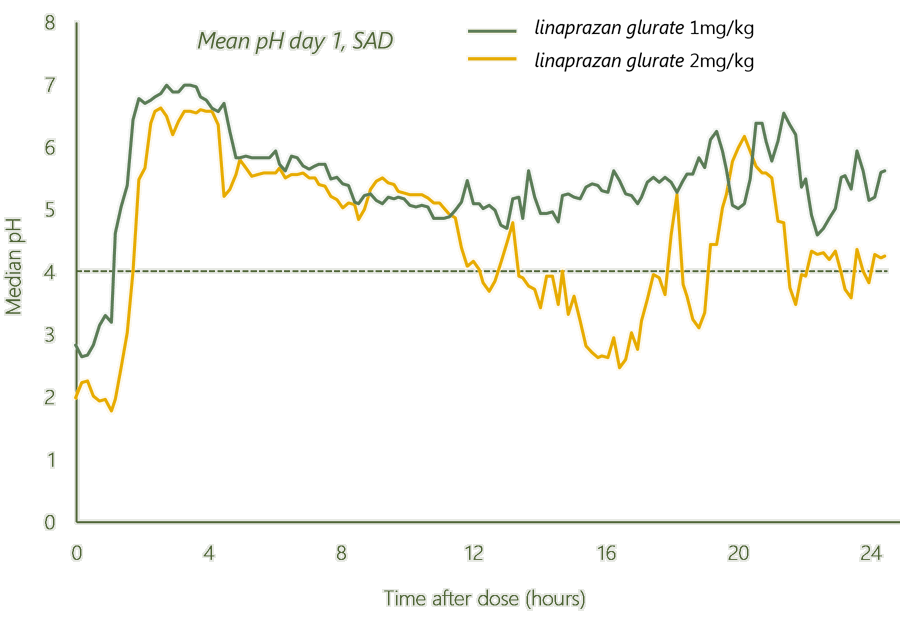
Dose-related pH control and an excellent biomarker are significant results from the completed Phase I studies.
Dose dependent pH control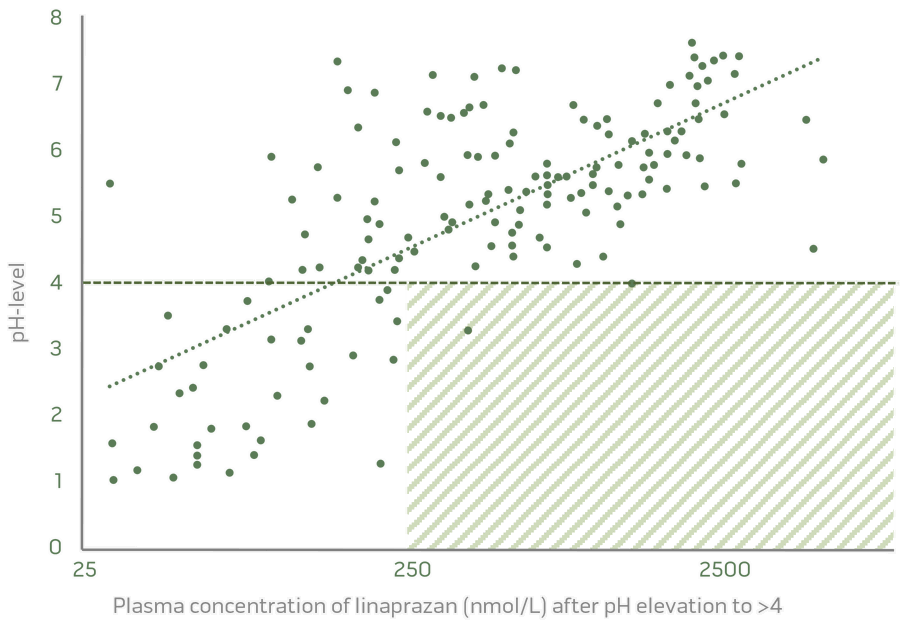
linaprazan glurate - Liquid formulation
Overview of SAD/MAD study
Study of single ascending dose (SAD) and Multiple ascending dose (MAD) performed in Sweden.
Primary objective and results
Linaprazan glurate was safe and well tolerated. No serious adverse event (SAE) occurred after dosing.
-
Dose related pharmacokinetics
-
Dose related acid inhibition and clear PK/PD relationship
-
No effect of food was detected
-
Study defined maximum dose was reached
-
Gastric acid inhibition was maintained over 24 hours at certain dose levels
Description
-
Single and multiple ascending dose
-
Liquid formulation
-
First-in-human design
-
Gov. identifier: NCT03105375
Endpoints
-
Frequency of adverse events, lab results and vital signs
-
Standard PK parameters
-
Dose limiting toxicity
-
PK/PD where PD is intragastric pH over 24h
Number of patients
-
In total, 28 subjects were dosed of which 9 subjects were dosed more than once
-
Performed by CTC Clinical Trial Consultants, Uppsala Sweden
Timetable
Initiated in February 2017. Final study report in February 2018.
The Phase II Study leed
In November 2022, Cinclus Pharma announced positive topline results from the phase II study LEED (Linaprazan glurate Erosive Esophagitis Dose Ranging).
The LEED trial was randomized, double-blind study conducted in the United States and Europe on patients with erosive esophagitis (eGERD). Patients were divided into two cohorts, one with patients having moderate to severe eGERD (Los Angeles (LA) classification grades C or D) and one with patients having milder eGERD (LA grades A or B) and preceding history of at least eight weeks healing course with proton pump inhibitor (PPI).
The primary objective of the study was to support dose selection of linaprazan glurate for the phase III program in eGERD, assessed as four-week endoscopic healing rates of eGERD, with safety and tolerability as secondary objectives. The number of patients needed for measuring efficacy was based on the patient cohort with moderate to severe eGERD.
In total, 248 patients were randomized to four weeks double-blind treatment with either one of the four dose levels of linaprazan glurate or the active comparator lansoprazole, a PPI in the approved eGERD healing dose, followed by four weeks open-label treatment with lansoprazole healing dose. Healing was defined as no presence of esophageal erosions, i.e., no erosive damage to the esophageal mucosa.
A retrospective central review of the endoscopy findings was performed after four weeks, and 162 patients with eGERD were available for evaluation of the primary endpoint. All enrolled 248 patients were included in the safety analysis.
For patients with moderate to severe eGERD, LA grades C or D, the highest four-week healing rate in a linaprazan glurate dosing group was 89%, compared to 38% in the lansoprazole group. While the study was not powered to demonstrate significance towards the comparator lansoprazole, the average healing rate in all C and D patients treated with linaprazan glurate was significantly higher than the healing rate in the lansoprazole group in a conservative post-hoc analysis (Fisher’s exact test, mean harmonic p-value <0.05).
For all patients treated with linaprazan glurate, the mean healing rate was 80% compared to 69% in the lansoprazole treated group. For patients with milder eGERD, LA grades A or B, the highest four-week healing rate in a linaprazan glurate dosing group was 91%, compared to 81% in the lansoprazole group. Linaprazan glurate was generally well tolerated and safety data was comparable to that of lansoprazole, with the most reported adverse event being COVID-19, occurring in 4% of the total study population.


