Most people can relate to discomfort from heartburn and regurgitation, two of the most common symptoms caused by GERD. Symptoms often occur during or after eating and drinking, which affects both motivation and the ability to enjoy social activities such as picnics, dinners, events, or just a cup of coffee with friends. More severe stages of GERD with the presence of esophageal erosions (eGERD) or ulcerations (complicated GERD) can be very painful.
Clinical Stage Pharma
Developing small molecules for the treatment of gastric acid related diseases.
The Next Leap Forward
Blockbuster Proton Pump Inhibitors (PPIs) such as Losec and Nexium have not been able to provide all gastroesophageal reflux disease (GERD) patients with sufficient disease control. The time has now come for Potassium-Competitive Acid Blockers (P-CABs) to take the next leap forward in the treatment of acid-related diseases. Cinclus Pharma’s clinical stage drug candidate linaprazan glurate takes a new approach to provide improved management of GERD and for the treatment of Helicobacter Pylori (H.Pylori) infection.

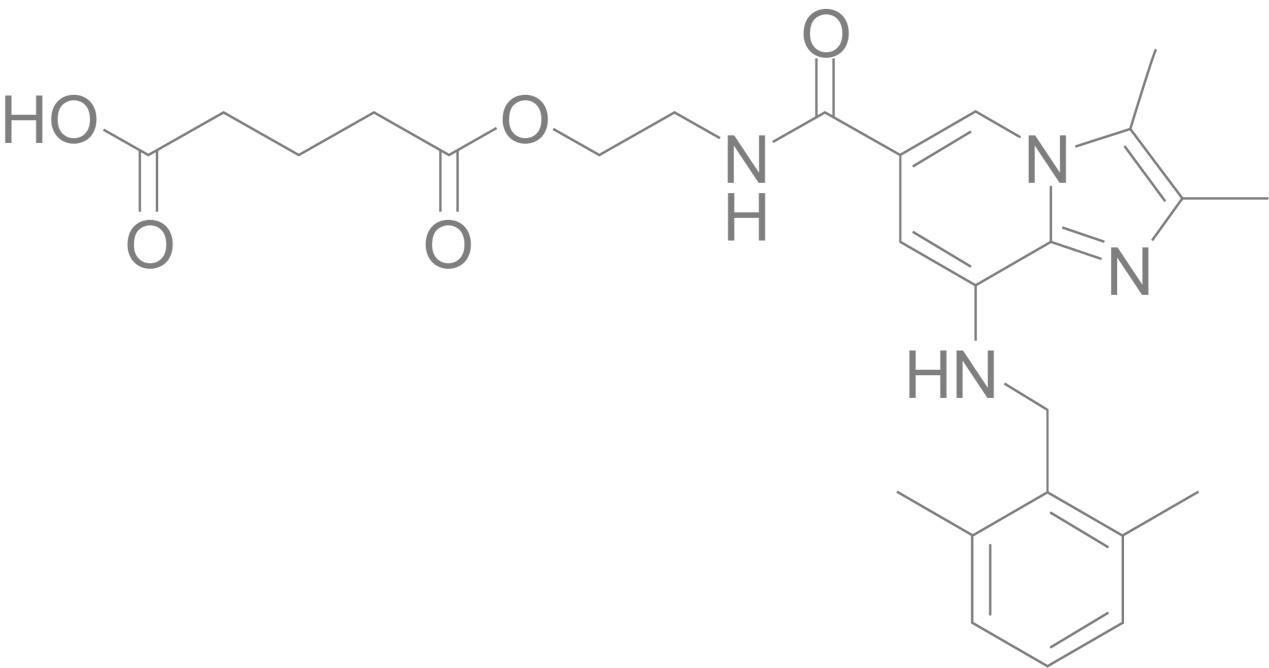
Linaprazan glurate
Built on world-leading industrial tradition, linaprazan glurate is a gastric acid blocker and therapy with great potential for healing GERD. Several clinical Phase I trials have been successfully completed and in 2022 the clinical Phase II trial was completed. Linaprazan glurate represents an improved mode of action with the potential for superior clinical efficacy and beneficial pharmacokinetic profile compared to PPIs.
In the spotlight

Kajsa Larsson, CMO, on the upcoming Phase III studies of linaprazan glurate
Highlights from the 2024 Annual Report: Interview with Kajsa Larsson. The study will involve around 500 patients from eight countries in Europe and North America, with up to 100 hospitals and clinics.

Watch the presentation from BioStock Global Forum
Now you can watch the presentation from BioStock Global Forum on May 21st, held by Christer Ahlberg, CEO of Cinclus Pharma.

Interview at Redeye about the partnership with Zentiva
Christer Ahlberg, CEO of Cinclus Pharma, was interviewed at Redeye about the partnership with Zentiva.

CHRISTER AHLBERG
CEO CINCLUS PHARMA



